Clinical Trial Collaboration Made Easy
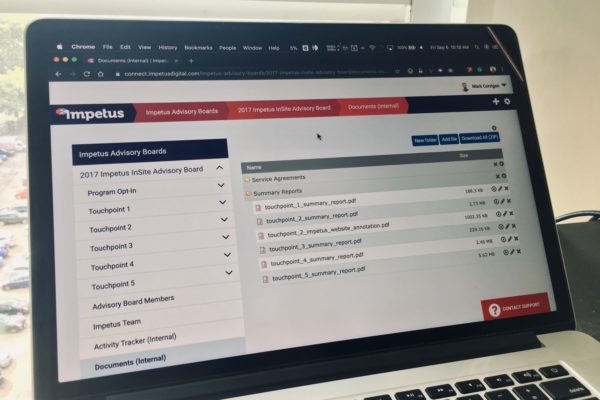
Impetus works with clinical operations throughout all phases of drug development, from early planning to final data dissemination. As examples, online advisory boards, working groups, and steering committees comprising investigators, healthcare professionals, patients, or other key stakeholders are effective means of reviewing or co-creating study protocols and for discussing the need for future clinical trials or subanalyses to address research gaps. For multicenter trials, frequent online touchpoints help improve communication and collaboration between study sites and between the sponsor and investigators.


Advisory boards, working groups, and steering committees
Clinical trial protocol development, amendments, evaluations, and gap analyses

Investigator engagement initiatives and site progress updates

Clinical trial support material development and site training

Publication planning, co-creation, or review
Multicenter trial communication and collaboration

Competitive landscape analyses
Development or reviews of target product profiles

Product life cycle planning

Research grants submission and approval programs

Virtual trial/remote trial hub

Virtual patient diary for adverse event and symptom monitoring